July 18, 2022 —Fresenius Kabiannounced today it will introduce a portfolio of genericcontrast mediaagents in the United States, starting immediately with the launch ofIodixanolInjection, USP, a product theU.S. Food and Drug Administration(FDA) lists as being in shortage nationwide.
Iodixanol Injection, USP is the first U.S. FDA-approved generic iso-osmolar, dimeric iodinated contrast media agent, which is used during diagnostic x-ray-based imaging such as computed tomography (CT) scans.
Iodixanoland other contrast media agents are in shortage across the U.S. due toCOVID-19related supply-chain disruptions. Fresenius Kabi is committed to preventing and mitigating shortages by working closely with its customers, suppliers, and the FDA, and by making long-term investments that support the supply chain of care in the United States.
造影剂对病人的诊断至关重要。据估计,美国每年有5000万例造影剂检查,全国有多达一半的医院受到造影剂短缺的影响。1
Iodixanol Injection, USP is bioequivalent and therapeutically equivalent toVisipaque这是费森尤斯卡比(Fresenius Kabi)独家提供的第一种通用碘造影剂,费森尤斯卡比是一家全球医疗保健公司,专门从事输液、输血和临床营养的药物和技术。
“Fresenius Kabi is pleased to help expand access to affordable, high-quality contrast media agents for the radiology community,” said John Ducker, president and CEO of Fresenius Kabi USA. “The approval and U.S. availability ofFresenius Kabi Iodixanol Injection, USP is expected to provide immediate relief to the current shortage. As a company committed to the purpose of ‘caring for life,’ we’re honored to help patients receive the timely care they need.”
Fresenius Kabi Iodixanol Injection, USP is an option for hospitals and clinics to use in the diagnosis of certain disorders of the brain, blood vessels, heart, kidneys, and other internal organs.2它不含防腐剂,装在一个聚合物瓶里。容器封口不使用天然橡胶胶乳。Fresenius Kabi Iodixanol Injection, USP is currently available in six presentations for intra-arterial and intravenous procedures:
270 mg Iodine per mL:
- 100 mL polymer bottle
- 150 mL polymer bottle
320 mg Iodine per mL:
- 50 mL polymer bottle
- 100 mL polymer bottle
- 150 mL polymer bottle
- 200 mL polymer bottle
“The U.S. availability of Iodixanol Injection, USP is the first offering from our generic radiology portfolio,” said Lindsey Thomas, senior vice president of Pharmaceutical Marketing at Fresenius Kabi USA and the company’s representative on theEnd Drug Shortage Alliance Board of Directors.“We are doing everything we can to accelerate product availability to help our customers during this acute contrast media agent shortage, including air shipping product. We are also actively working to bring additional affordable contrast agents to U.S. clinicians to help ensure patient access to essential diagnostic imaging procedures.”
Indications and Usage
Iodixanol injection is a radiographic contrast agent indicated for the following:
Intra-arterial Procedures
Adults and pediatric patients 12 years of age and over
- Intra-arterial digital subtraction angiography (270 mg Iodine/mL and 320 mg Iodine/mL).
- Angiocardiography(左心室造影和选择性冠状动脉造影)、周围动脉造影、内脏动脉造影和大脑动脉造影(320 mg碘/mL)。
Pediatric patients less than 12 years of age
- Angiocardiography, cerebral arteriography, and visceral arteriography (320 mg Iodine/mL).
Intravenous Procedures
Adults and pediatric patients 12 years of age and over
- Computed tomography(CT) imaging head and body (270 mg Iodine/mL and 320 mg Iodine/mL).
- 排泄尿路造影(270mg碘/mL和320mg碘/mL)
- Peripheral venography (270 mg Iodine/mL).
- Coronary computed tomography angiography(CCTA) to assist diagnostic evaluation of patients with suspected coronary artery disease (320 mg Iodine/mL).
Pediatric patients less than 12 years of age
- 头部和身体的CT显像(270 mg碘/mL)。
- Excretory urography (270 mg Iodine/mL).
Important Safety Information
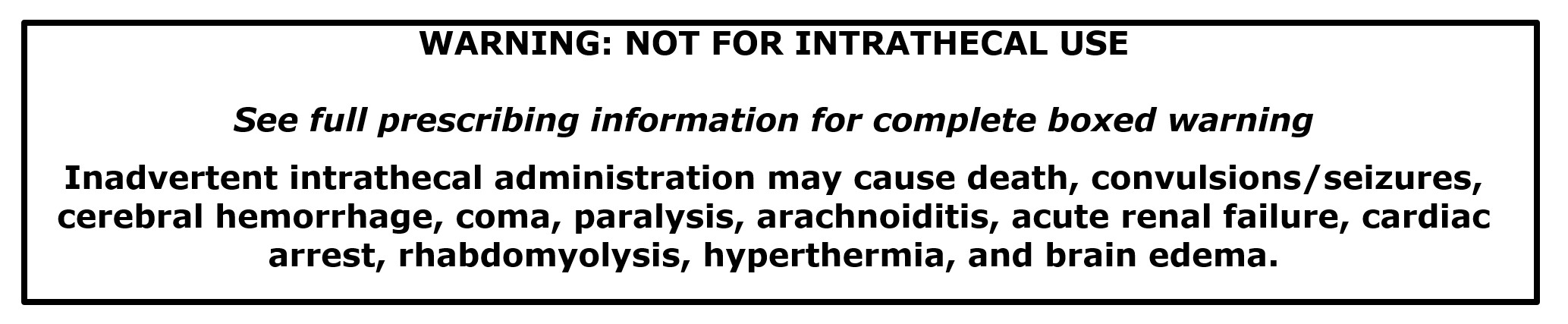
Iodixanol is contraindicated for intrathecal use.
- Hypersensitivity Reactions: Life-threatening or fatal reactions can occur. Always have emergency equipment and trained personnel available.
- 造影剂引起的急性肾损伤:可发生包括肾功能衰竭在内的急性损伤。尽量减少剂量,保持足够的水合作用,以减少风险。
- Cardiovascular Adverse Reactions: Hemodynamic disturbances including shock and cardiac arrest may occur during or after administration.
- Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age: Monitor these patients for thyroid function abnormalities and treat as clinically needed.
Adverse Events: Most common adverse reactions (incidence greater than 0.5%) in adult patients after iodixanol injection: Discomfort, warmth, pain; Cardiovascular: angina. Gastrointestinal: diarrhea, nausea, vomiting. Nervous System: agitation, anxiety, insomnia, nervousness, dizziness, headache, migraine, unusual skin sensations, sensory disturbance, fainting, sensation of spinning. Skin: itchy rash, severe itching, hives. Special Senses: Smell, taste, and vision alteration. Pediatric patients experienced similar adverse reactions.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
- Lactation: A lactating woman may pump and discard breast milk for 10 hours after iodixanol administration.
- Geriatrics: Exercise caution in dose selection for elderly patients.
本重要安全信息不包括安全有效地使用碘沙醇注射液USP所需的所有信息。Please see full prescribing information, including BOXED WARNING, for Iodixanol Injection, USP atwww.fresenius-kabi.com/us.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
For more information:www.fresenius-kabi.com/us
Related Content on Gadolinium Concerns
Global Contrast Media Shortage: Strategies for Conservation
AJR Publishes Best Practices for Iodinated Contrast Media Shortage
Voluntary Dismissal of Chuck Norris Gadolinium Case Involving Bracco
VIDEO: How Serious is MRI Gadolinium Retention in the Brain and Body?An interview with Max Wintermark, M.D.
VIDEO “Big Concerns Remain for MRI Gadolinium Contrast Safety at RSNA 2017,”An interview with Emanuel Kanal, M.D.
Radiology Has Failed to Properly Assess or Track MRI Gadolinium Contrast Safety
Recent Developments in Contrast Media
FDA Committee Votes to Expand Warning Labels on Gadolinium-Based Contrast Agents
European Medicines Agency Issues Update on Gadolinium Contrast Agents


 August 10, 2022
August 10, 2022