June 8, 2022 —Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, today announced completion of patient accrual in itsPhase 3 REVELATEclinical trial of18F-fluciclovine, apositron emission tomography(PET) imaging radiopharmaceutical being studied for potential use in detecting recurrent brain metastases after radiotherapy. TheREVELATE studyis a prospective Phase 3, multi-center, single-arm imaging study being conducted in the United States.
Note:18F-fluciclovine is an approved molecular imaging radiopharmaceutical for use in PET imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment. The safety and efficacy of18F-fluciclovine PET imaging for the detection of recurrent brain metastases has not been established.
”Expanding our18F-fluciclovine franchise into neuro-oncology is part of the overall growth strategy for Blue Earth Diagnostics, and completion of Phase 3 patient accrual sets a major milestone in our development plan,” saidDavid E. Gauden, D.Phil., Chief Executive Officer. “We look forward to receiving the clinical results from REVELATE, and to presenting results of the Phase 2 PURSUE study at upcoming scientific meetings later this year. Additionally, we wish to thank the patients, physicians and clinical trial sites who worked closely with us to complete enrollment despite the many challenges presented by the COVID-19 pandemic. In line with our mission to develop novel PET radiopharmaceuticals to inform the management and care of patients with cancer, we are hopeful that our efforts may help patients with recurrentmetastatic brain cancer.”
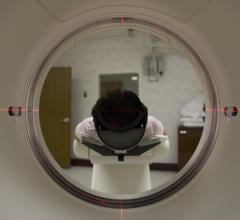
”Radiation therapy is a mainstay of treatment for brain metastases which provides effective tumor control but can result in radiation necrosis,” saidSamuel T. Chao, MD, Department of Radiation Oncology,Cleveland Clinic; Professor at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, Ohio; and Coordinating Investigator on the REVELATE Phase 3 study. “Serial magnetic resonance imaging (MRI) is often used to monitor patients after treatment. However, physicians face challenges in diagnosing and managing suspicious lesions found upon post-treatment surveillance, as they may represent tumor recurrence or treatment-related changes such as radiation necrosis. Significant progress has been made in diagnostic imaging modalities to assist in differentiating these entities, among them the use of amino acid-based PET radiopharmaceuticals. The Phase 3 REVELATE trial is designed to investigate the diagnostic performance of amino acid18F-fluciclovine PET imaging as a potential decision-making aid in assessing the status of a patient’s disease.”
“传统MRI的局限性在神经肿瘤反应评估(RANO)小组制定的指南和建议中得到了承认。RANO/PET工作组在2019年的建议中提到了氨基酸PET放射性药物在区分放疗后的脑组织变化和复发性脑转移方面的潜在用途。MBBS、MRCP、FRCR、d.f ill, Eugene J. Teoh说:“现有数据主要来源于单中心回顾性研究,我们再次呼吁进行前瞻性多中心研究,以验证这些观察结果。”蓝色地球诊断公司的首席医疗官”18F-Fluciclovine holds potential clinical utility for the detection of other cancers besides recurrent prostate cancer. As an amino acid-based PET radiopharmaceutical,18f -氟氯洛万旨在可视化恶性肿瘤中氨基酸转运增加的情况,我们急切地等待REVELATE和PURSUE临床试验的结果。”
About the REVELATE and PURSUE Clinical Trials in Brain Metastases
Blue Earth Diagnostics has two clinical studies investigating the use of18F-fluciclovine PET in the detection of recurrent brain metastases. The REVELATE study (“Study to Establish the Diagnostic Performance of18F-fluciclovine PET in Detecting Recurrent Brain Metastases”) is an open-label, single-arm, single-dose, prospective, multi-center Phase 3 study designed to establish the diagnostic performance of18F-fluciclovine PET in detecting recurrent brain metastases after radiation therapy. The primary endpoint of the REVELATE study is to assess the Negative Percent Agreement (NPA, equivalent to specificity) and Positive Percent Agreement (PPA, equivalent to sensitivity) of18F-fluciclovine PET in detecting recurrent brain metastases on a patient level. Secondary endpoints will assess the Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of18F-fluciclovine PET for detecting recurrent brain metastases, among others. The Phase 2 PURSUE trial is designed to establish image interpretation criteria for18F-fluciclovine PET in detecting recurrent brain metastases. Further information about these trials can be found onwww.clinicaltrials.gov(REVELATE,NCT04410133, PURSUE,NCT04410367).
About18F-Flucivlovine PET and Recurrent Brain Metastases
18F-flucivlovine PETis a novel diagnostic imaging radiopharmaceutical for PET imaging to visualize the increased amino transport that occurs in malignant tumors. It consists of a synthetic amino acid that is preferentially taken up by cancer cells compared with surrounding normal tissues and is labeled with the radioisotope18F for PET imaging.18F-flucivlovine is under investigation by Blue Earth Diagnostics for potential use in adults for the detection of recurrent brain metastases in patients who have previously undergone radiation therapy.18F-fluciclovine is approved by the U.S. Food and Drug Administration (FDA) and in the EU for PET imaging in men with recurrent prostate cancer.18F-fluciclovine was invented at Emory University, in Atlanta, Ga., with much of the fundamental clinical development carried out by physicians at Emory University’s Department of Radiology and Imaging Sciences. Blue Earth Diagnostics licensed18F-fluciclovine from GE Healthcare and is investigating the molecule for other potential cancer indications, including in neuro-oncology.
For more information:www.blueearthdiagnostics.com
Related content:
Blue Earth Diagnostics Announces Key Results from Phase 3 SPOTLIGHT Study of 18F-rhPSMA-7.3


 August 10, 2022
August 10, 2022