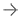
Getty Images
July 20, 2022 —Blue Earth Diagnostics, aBraccocompany and recognized leader in the development and commercialization of innovativePETradiopharmaceuticals, today shared news of the publication of two independent, retrospective studies evaluating the use ofAxumin(fluciclovine F 18)PET imagingin men with biochemical recurrence ofprostate cancerwho were undergoing androgen deprivation therapy (ADT). The first study of 320 men, 68 of whom were on ADT, was published inUrologic Oncology.结果显示,接受ADT的患者与未接受ADT的252例患者在Axumin PET显像性能上无差异。Both populations had a whole body Axumin positivity rate of 82%, consistent with the patient population and positivity rates of data presented to the U.S. Food and Drug Administration (FDA) for approval of Axumin. The second study, published in Tomography, assessed 71 patients with biochemical recurrence ofprostate canceron ADT for three or more months. It found that there was no difference in Axumin performance and that the length of time on ADT did not influence Axumin detection rates. Axumin, a novel amino acid-based radiopharmaceutical, is FDA-approved for PET imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
“Physicians face challenges in localizing recurrent disease in patients with prostate cancer who experience biochemical failure while on ADT,” saidBital Savir-Baruch, MD, Chief of Nuclear Medicine,University of Arizona College of Medicine亚利桑那州图森市。and affiliated withLoyola University、芝加哥、生病。“已知ADT会影响前列腺特异性膜抗原(PSMA)受体的表达,并能抑制雄激素敏感前列腺癌患者对胆碱类药物的摄取。ADT对18f -氟氯洛酮成像的影响在临床实践中还没有很好的文献记录。洛约拉大学的一项大型回顾性研究探讨了18f -氟氯洛韦对复发性前列腺癌患者疗效的影响。Loyola研究的这些结果表明,接受ADT治疗的前列腺癌生化复发患者与未接受ADT治疗的患者有相似的18f -氟氯lovine阳性率,并且在两组中,阳性率随着PSA水平的增加而增加。”
“We are pleased to note the publication of these clinically relevant studies that illustrate the ongoing interest of the physician community in the role that Axumin molecular imaging can have in guiding informed patient care for men with biochemical recurrence of prostate cancer,” saidDavid E. Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “Blue Earth is the recognized leader in diagnostic PET prostate cancer imaging, and we continue to advance scientific knowledge about the proven clinical utility of Axumin (seeAxumin full Indication and Important Information).Our prospective FALCON and LOCATE studies demonstrated that AxuminPET/CTlocated recurrent disease in the majority of men in the study, which frequently resulted in significant changes to their management plans for biochemical disease recurrence. Notably,Emory University’s independent EMPIRE-1 study (NCT01666808), published inThe Lancet研究表明,采用Axumin PET成像的治疗可显著提高复发前列腺癌男性患者3 - 4年的无事件生存期。这些结果包括30例(38%)复发性前列腺癌患者,他们在Axumin组中接受ADT治疗。”
Dr. Gauden continued, “Axumin PET imaging has informed healthcare decisions for more than 165,000 men with recurrent prostate cancer across the United States, where it is available at more than 1,350 imaging centers and widely reimbursed. Blue Earth Diagnostics is committed to helping patients through innovative diagnostic solutions that empower the evolution of care for men with recurrent prostate cancer, and we look forward to helping even more patients in the future.”
Highlights of the publications
“Effect of Hormonal Therapy on 18F-Fluciclovine PET/CT in the Detection of Prostate Cancer Recurrence, Localization of Metastatic Disease and Correlation with Prostate-specific Antigen”
(DOI:https://doi.org/10.1016/j.urolonc.2022.05.018)
The study was designed to explore the impact of androgen deprivation therapy (ADT) on the performance of 18F-fluciclovine. A retrospective analysis was conducted to compare the 18F-fluciclovine PET/CT positivity rate in 320 patients with biochemical recurrence of prostate cancer receiving ADT at the time of the scan with the rate achieved in those not receiving ADT. For each group, the number of positive 18F-fluciclovine PET/CT scans (positivity rate) was evaluated for the whole body, prostate/bed, and extraprostatic regions, and rates were correlated with PSA. The 18F-fluciclovine positivity rate was analyzed at the patient level, in the prostate/bed region and at each extraprostatic site (pelvic lymph nodes, extrapelvic lymph nodes, bone and soft tissue or other metastatic sites). Positivity rates were stratified according to whether or not the patient was receiving ADT at the time of scan.
在18f氟氯氯胺扫描时,68/320(21%)例患者使用ADT,而252/320(79%)例患者未使用ADT。ADT组中位Gleason评分为8(范围6-10),非ADT组中位Gleason评分为7(范围6-10)。总的来说,ADT组和非ADT组之间的阳性率没有统计学意义。全组阳性率(ADT vs.非ADT)为82% (56/68)vs. 82%(206/252),前列腺/床阳性率为57% (39/68)vs. 60%(152/252),前列腺外区阳性率为60% (41/68)vs. 53%(133/252)。ADT组与非ADT组在不同PSA水平上无显著差异。作者指出了该研究的某些局限性,其中包括其回顾性和缺乏组织病理学数据,以及ADT治疗时间未知。正如摘要中所述,作者得出结论,“18f -氟氯洛韦PET/CT检测前列腺癌复发不受ADT的显著影响,这表明在接受ADT的PSA可检测患者中,使用18f -氟氯洛韦进行疾病定位是可行的。””1
The manuscript, “Effect of Hormonal Therapy on 18F-Fluciclovine PET/CT in the Detection of Prostate Cancer Recurrence, Localization of Metastatic Disease and Correlation with Prostate-specific Antigen” was published online on June 21, 2022, in Urologic Oncology. It will also appear in an upcoming print issue. Authors on the manuscript were: Jad El Bulbul, Abdulrahman Hashem, Damian Grybowski, Cara Joyce, Essam Rashad, Medhat S. Gabriel, Robert H. Wagner, and Bital Savir-Baruch. All authors are affiliated with Loyola University Medical Center or its Health Sciences Campus, Maywood, Ill. Dr. Savir-Baruch is now affiliated with the University of Arizona College of Medicine, Tucson, Ariz. as well as Loyola University.
“Effect of Androgen Deprivation Therapy on the Results of PET/CT with 18F-Fluciclovine in Patients with Metastatic Prostate Cancer”
(DOI:https://doi.org/10.3390/tomography8030120).
The study was designed to investigate the impact of concurrent ADT on disease detection with 18F-fluciclovine PET in patients with metastatic prostate cancer following primary therapy. Data from 71 patients who had been receiving ADT for at least 3 months at the time of undergoing an 18F-fluciclovine PET/CT scan were retrospectively reviewed. The reasons for the PET/CT scan were rising PSA (n=58), staging of advanced disease (n=4) or therapeutic monitoring (n=9).
Malignant lesions with increased uptake of 18F-fluciclovine were detected in 60/73 (82%) of the scans; 33 (45%) had lesions in the prostate/bed and 46 (63%) in extraprostatic sites. Patients received ADT for a median of 2 years pre-scan. The time on ADT did not influence detection. The detection rates were 89% for patients who had received ADT for < 1 year, 63% for a period of 1 - <2 years, 83% for 2 - 4 years, 78% for > 4 - 10 years and 67% for a treatment period of >10 years. The authors cited certain limitations to the study, including its retrospective nature and lack of histopathological data, and the inability to compare 18F-fluciclovine scans while patients were on ADT and after withdrawal. As stated in the abstract, the authors concluded that “18F-fluciclovine detected recurrent or metastatic lesions in 82% of patients with prostate cancer receiving ADT. The rates achieved are consistent with widely reported data for 18F-fluciclovine PET/CT, suggesting that withdrawal of ADT before scanning is not necessary.”2
The manuscript, “Effect of Androgen Deprivation Therapy on the Results of PET/CT with 18F-Fluciclovine in Patients with Metastatic Prostate Cancer” was published in Tomography on June 3, 2022. The manuscript will also appear in an upcoming print issue. Authors on the manuscript were: Tore Bach-Gansmo, Katrine Korsan and Trond Velde Bogsrud. Dr. Bach-Gansmo and Katrine Korsan are affiliated withOslo University Hospital; Oslo, Norway. Dr. Bogsrud is affiliated with Oslo University Hospital and Aarhus University Hospital, Aarhus, Denmark.
Indication and Important Safety Information About Axumin
INDICATION
Axumin (fluciclovine F 18) injection is indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment.
IMPORTANT SAFETY INFORMATION
Image interpretation errors can occur with Axumin PET imaging. A negative image does not rule out recurrent prostate cancer and a positive image does not confirm its presence. The performance of Axumin seems to be affected by PSA levels. Axumin uptake may occur with other cancers and benign prostatic hypertrophy in primary prostate cancer. Clinical correlation, which may include histopathological evaluation, is recommended.
Hypersensitivity reactions, including anaphylaxis, may occur in patients who receive Axumin. Emergency resuscitation equipment and personnel should be immediately available.
Axumin use contributes to a patient’s overall long-term cumulativeradiation exposure, which is associated with an increased risk of cancer. Safe handling practices should be used to minimize radiation exposure to the patient and health care providers.
Adverse reactions were reported in ≤ 1% of subjects during clinical studies with Axumin. The most common adverse reactions were injection site pain, injection site erythema and dysgeusia.
For more information:www.blueearthdiagnostics.com
Related content:
Blue Earth Diagnostics Announces Key Results from Phase 3 SPOTLIGHT Study of 18F-rhPSMA-7.3


 August 10, 2022
August 10, 2022