February 14, 2019 — Smart technology company Synopsys recently announced the release of the Synopsys Simpleware ScanIP Medical edition for3-D printingandvisualization. This new version of Simpleware ScanIP comes with U.S. Food and Drug Administration (FDA) 510(k) clearance, and CE and ISO 13485:2016 certification as a medical device.
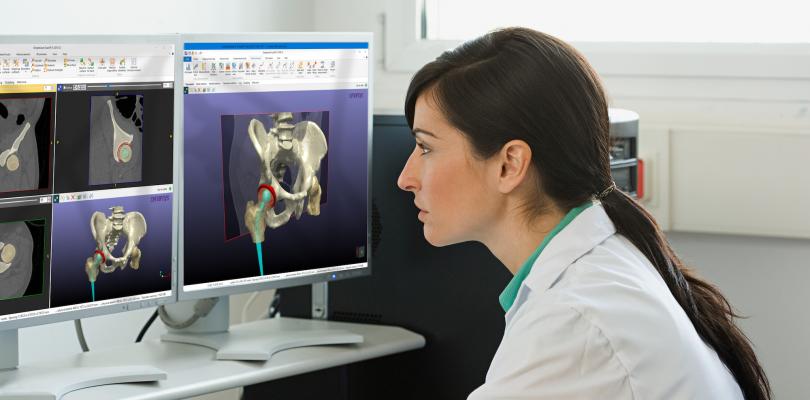
According to the company, Simpleware ScanIP Medical is the ideal choice for those working with 3-D imaging to create medical devices for pre-clinical workflows such as implant design and patient-specific planning. Developed and maintained to international standards for medical device software, Simpleware ScanIP Medical comes with all the features of ScanIP but is specifically intended for medical usage. Users can process medical imaging data and export output files to simulate/evaluate pre-surgical treatment options.
Workflow highlights of the software include:
Import and anonymize patient DICOM tags from the picture archiving and communication system (PACS) server. The software is DICOM 3.0 compliant;
Combine computer-aided detection (CAD) and imaging data for patient-specific analysis;
Rapidly segment and process medical images with easy-to-use interface;
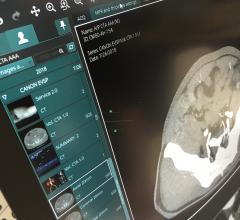
Obtain reliable data for complex anatomical analysis using measurement and statistics tools; and
Export geometrically accurate models for pre-surgical planning
Key benefits associated with Simpleware ScanIP Medical include:
Intuitive, fully supported user interface tested by medical professionals;
Compliant with privacy standards for handling patient data;
Achieve ideal surgical outcomes based on insights from imaging data;
Improve understanding of patient anatomy when designing unique medical devices;
Streamline software resources with complete medical image processing platform for the R&D workstation, radiology department or other clinical work environments; and
Build confidence in surgical decision-making by simplifying time-consuming workflows.
For more information:www.synopsys.com

 May 11, 2022
May 11, 2022