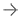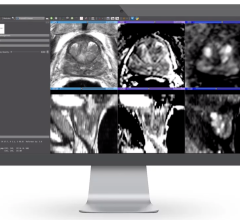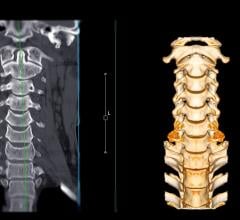
July 13, 2022 —Royal Philips, a global leader in health technology, announced its SmartSpeed artificial intelligence (AI) powered MR acceleration software has received U.S. Food and Drug Administration (FDA) 510(k) clearance. Adding advanced AI data collection algorithms toPhilips’ existing Compressed SENSE MR acceleration engine,Philips SmartSpeeddelivers higher image resolution with 3 times faster scan times [1] and virtually no loss in image quality, representing a major step forward in diagnostic confidence and MR department productivity.
With personalized treatment for complex diseases such ascancerincreasing the need for high-confidence precision diagnoses, coupled with soaring caseloads due to aging populations and high levels ofclinician burnout,radiologydepartments are under increasing pressure to improve performance, productivity, and profitability.
“Philips’ AI-based SmartSpeed reconstruction is the new benchmark among acceleration techniques for us. It improves on the company’s existingCompressed SENSEin all aspects and allows a reduction in scan times with excellent image quality and diagnostic confidence,” said Dr.Grischa Bratke, radiologist and expert in musculoskeletal imaging at theUniversity Hospital of Cologne, Cologne, Germany.
Philips SmartSpeed, the latest addition to Philips’ expanding portfolio of AI-driven, smart, connected imaging and smart workflow solutions, helps achieve the Quadruple Aim of improving diagnostic outcomes, enhancing patient and staff experiences, and reducing healthcare costs overall.
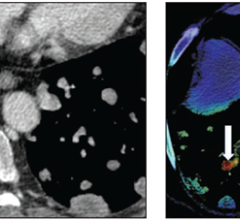
This latest AI-powered MR software increases resolution up to 65% and achieves up to 3 times faster scanning times compared to conventional MR scans [1]. Coupled with its support for 97% of current clinical protocols [2] – including advanced contrasts and diffusion weighted imaging, and even quantitative imaging such at T1 or T2 mapping for brain, liver, heart or musculoskeletal (MSK) imaging – Philips SmartSpeed helps increase diagnostic confidence in a wide range of patients, including the most complex cases. The unique deep learning technology of Philips SmartSpeed is applied at the source of the MR signal and seamlessly integrated with Philips leading Compressed SENSE speed engine to deliver higher resolution and high signal-to-noise ratio needed for detailed images, small lesion detection and enhanced diagnosis.
Award-winning AI technology of Philips SmartSpeed
Philips SmartSpeed uses an award-winning AI reconstruction algorithm [3] at the front-end of the MR signal to remove noise and preserve details while enabling k-space data consistency check for trustworthy AI. The unique Compressed SENSE adaptive AI technology used in Philips SmartSpeed, which was co-developed by Philips and several of its academic partners, won the 2019fastMRI Challengehosted by Facebook AI research and New York Langone Health.
“By taking the Quadruple Aim as a starting point to develop and deploy AI solutions, we have a tremendous opportunity to address the inefficiencies and growing demand that our customers and partners face every day,” saidKees Wesdorp, Chief Business Leader of Precision Diagnosis at Philips. “Powered by AI, our leading technologies and smart connected imaging solutions help turn data into actionable insights to increase diagnostic confidence and help improve clinical outcomes for patients.’’
Philips Portfolio of Smart, Connected Imaging and Intelligent Workflow Solutions at ECR 2022
Philips SmartSpeed joins the growing number of Philips AI powered workflow innovations that are available across the portfolio ofsmart Philips MR systemsthat will be showcased at the upcoming 2022 European Congress of Radiology(ECR 2022), including the recent European introduction of the smart connected 3T MR system,Philips MR 7700and the company’s latestMR 53001.5T helium-free in operations MR system. Each of these systems uses intelligent software to automate tasks and help relieve the burden on busy radiology staff and departments.
VisitPhilips at ECR 2022for more information on Philips’ AI-powered Precision Diagnosis suite of solutions, featuring smart connected imaging systems and integrated radiology workflow solutions, designed to increase clinical confidence and diagnostic outcomes.
For more information:www.usa.philips.com
References:
Philips SmartSpeed is FDA cleared but not CE marked, and not yet available for delivery in all countries.
[1] Compared to Philips SENSE
[2] Average measured across a sample of sites from Philips MR installed base
[3] Adaptive-C-SENSE-Net technology is the winner of Fast MRI Challenge hosted by Facebook AI research and New York Langone Health


 August 10, 2022
August 10, 2022