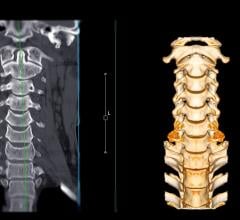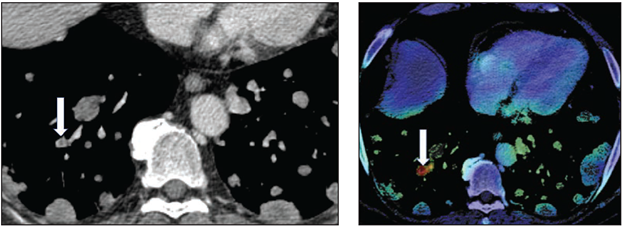
Left: Axial image shows central nonocclusive filling defect in right lower lobe lateral subsegmental pulmonary artery (arrow). Examination classified positive for iPE by AI but negative for iPE by clinical report. Adjudication process used for reference standard determination classified examination as positive for iPE. Right: Activation map shows location corresponding with iPE identified by AI (arrow).
July 21, 2022 —According to ARRS’ American Journal of Roentgenology (AJR), anAItool for detection of incidental pulmonary embolus (iPE) on conventional contrast-enhanced chestCTexaminations had high NPV and moderate PPV, even finding some iPEs missed by radiologists.
“该人工智能工具的潜在应用包括作为第二读取器,帮助检测额外的iPE,或作为工作列表分类工具,允许更早的iPE检测和干预。”wrote lead investigatorKiran Batrafrom theUniversity of Texas Southwestern Medical Centerin Dallas. “Various explanations of misclassifications by the AI tool (both false positives and false negatives) were identified, to provide targets for model improvement.”
Batra and colleagues’ retrospective study included 2,555 patients (1,340 women, 1,215 men; mean age, 53.6 years) who underwent 3,003 conventional contrast-enhanced chest CT examinations between September 2019 and February 2020 at Parkland Health in Dallas, TX. Using an FDA-approved, commercially available AI tool (Aidoc, New York, NY) to detect acute iPE on the images, a vendor-supplied natural language processing algorithm (RepScheme, Tel Aviv, Israel) was then applied to the clinical reports to identify examinations interpreted as positive for iPE.
Ultimately, the commercial AI tool had NPV of 99.8% and PPV of 86.7% for detection of iPE on conventional contrast-enhanced chest CT examinations (i.e., not using CTpulmonary angiographyprotocols). Of 40 iPEs present in the team’s study sample, 7 were detected only by the clinical reports, and 4 were detected only by AI.
Noting that both the AI tool and clinical reports detected iPEs missed by the other method, “the diagnostic performance of the AI tool did not show significant variation across study subgroups,”the authors of this AJR article added.
For more information:arrs.org


 August 10, 2022
August 10, 2022