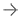![Royal Philips announced new additions to its CT imaging portfolio at the Radiological Society of North America (RSNA) annual meeting. The new CT 5100 – Incisive – features CT Smart Workflow [1], a comprehensive suite of artificial intelligence* (AI) enabled capabilities designed to accelerate CT workflows, enhance diagnostic confidence, and maximize equipment up-time, helping imaging services to enhance patient outcomes, improve department efficiency, reduce operational costs, and meet ambitious financial o](http://www.alohadebbie.com/sites/default/files/styles/content_large/public/Screen%20Shot%202022-01-06%20at%209.55.32%20AM.png?itok=BJ5uw22n)
January 6, 2022 —Royal Philipsannounced new additions to itsCTimaging portfolio at theRadiological Society of North America(RSNA) annual meeting. The new CT 5100 – Incisive – features CT Smart Workflow [1], a comprehensive suite of artificial intelligence* (AI) enabled capabilities designed to accelerate CT workflows, enhance diagnostic confidence, and maximize equipment up-time, helping imaging services to enhance patient outcomes, improve department efficiency, reduce operational costs, and meet ambitious financial objectives.
CT 5100 – Incisive – with CT Smart Workflow [1] includes Philips’Tube for Lifeguarantee, which over the lifetime of the scanner can potentially lower operating expenses by an estimated USD 420,000 [2][3]. This newest CT innovation from Philips also provides access to Philips’Technology Maximizerprogram, which provides users with the latest software and hardware updates as they are released.
“With the combination of CT 5100 – Incisive – and CT Smart Workflow, we have embedded AI into the tools that radiology departments use every day so they can apply their expertise to the patient, rather than unnecessary distractions associated with the CT imagingitself,” said Frans Venker, General Manager of Computed Tomography at Philips. “By automating many of the process-related obstacles to CT imaging performance, we aim to clear the way for precision in dose, speed, and image quality that will help imaging departments to meet their financial, clinical, and operational goals.”
Improving the CT experience with smart clinical decision-making CT 5100 – Incisive – with CT Smart Workflow adds to Philips’ growing suite of AI-enabled smart workflow solutions, including therecently launchedMR 5300 1.5T系统和新的MR 7700 [4] 3.0T系统。最近引入的这些智能系统都旨在通过自动化许多耗时的程序任务(放射科医生、技术人员和工作人员传统上必须手工完成的任务)来支持提高效率、操作员的一致性和图像采集点的诊断信心。
CT Smart Workflow is the latest in a continuous program of performance enhancements forPhilips’ market-leading Incisive CT platform, which includes a newly-designed patient table to accommodate bariatric patients; OnPlan gantry controls that demonstrate a 19% [5] reduction in time to results; and the company’s process improvement services Enterprise Performance Analytics – PerformanceBridge. The CT 5100 – Incisive – with CT Smart Workflow demonstrates how integrated, AI-driven solutions connect clinical intelligence and operational data across the radiology enterprise to advance precision diagnosis and treatment.
For more information:www.philips.com
References:
[1] CT Smart Workflow pending 510(k). Not for sale in the USA
[2] Life of the product is defined by Philips as 10 years. Tube for Life guarantee availability varies by country. Please contact your local Philips sales representative for details
[3] Actual operating costs for customers vary significantly because many variables exist (such as CT make and model, hospital/ imaging center size, case mix, system usage). The potential savings identified estimates the avoidance of purchasing replacement tubes over a 10-year useful life of a CT system, based on an average selling price of $140,000 per replacement tube and estimated tube life of 3 years. There can be no guarantee that all customers will achieve this result. Tube for Life guarantee availability varies by country
[4] Pending 510(k) clearance. Not available for sale in the USA
[5] Based on a study Performed at Oz Radiology Group, Queensland, Australia. Results from case studies are not predictive of results in other cases. Results in other cases may vary
*According to the definition of AI from the EU High-Level Expert Group


 August 11, 2022
August 11, 2022