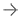July 1, 2022 —Insightec, a global healthcare company dedicated to using acoustic energy to transform patient care, today announced that it has receivedFDAapproval for aninvestigational device exemption(IDE) for a clinical comparative study of the Exablate Prostate system used to treatdiseased prostate tissue. This study will evaluate the safety and efficacy of focal treatment using high intensity focused ultrasound when compared to active surveillance in men living withprostate cancer.
TheInsightec Exablate Prostate systemuses sound waves to ablate, or destroy, targeted tissue in the prostate. The treatment is performed under Magnetic Resonance Imaging (MRI) guidance for high resolution visualization of the patient’s anatomy for precise targeting and real-time temperature monitoring. The single session treatment does not require incisions and allows patients to quickly return to normal activity with minimal complications.
“We are excited to continue this important research that can impact on the standard of care for prostate cancer treatment,” saidBehfar Ehdaie, MD, MS, a urologic surgeon atMemorial Sloan Kettering Cancer Center, and principal investigator for the study. “Exablate Focused Ultrasound has been shown to provide an accurate, safe, and effective option to engage the prostate gland directly in select patients based on 2-years biopsy outcomes. The new trial will build on this success and help further enhance treatment options.”
“At Insightec, we are committed to the next generation of prostate cancer research and patient care,” saidMaurice R. Ferré, MD他是Insightec的首席执行官兼董事会主席。“通过技术创新和医疗进步,在过去十年中,前列腺治疗取得了重大进展,但我们还没有完成。我们这项研究的目标是证明Exablate前列腺疗法的临床益处,并为患者提供改善生活质量的机会。”
A previous Insightec-sponsored clinical trial led by Memorial Sloan Kettering Cancer Center for the Exablate Prostate system reported minimal damage to adjacent structures and low rates of impact on potency and continence, supporting function and quality of life for patients. The new comparative study builds on the evidence of this clinical trial and aims to further enhance prostate treatment options and improve clinical outcomes.
The Insightec Exablate Prostate system received 510(k) FDA clearance in November 2021, making way for the system to be offered to patients in a commercial facility and for further clinical studies. In January 2022, the system was used to treat prostate disease in its first US commercial patient.
这项新研究的结果将确定病灶治疗在延迟和避免前列腺癌男性根治治疗中的作用,并支持扩大该技术的临床应用,并通过保险报销增加患者获得治疗的机会。
For more information:www.insightec.com
Related prostate cancer content:
SNMMI Applauds FDA Approval of New Metastatic Prostate Cancer Treatment
PSMA PET Validates EAU Classification System to Determine Risk of Prostate Cancer Recurrence
VIDEO: MRI-Linac and PSMA PET Imaging Technologies Aids Therapy at GenesisCare
FDA Approves First Commercially Available PSMA PET Imaging Agent for Prostate Cancer
PSMA-Targeted Radiotracer Pinpoints Metastatic Prostate Cancer Across Anatomic Regions
Metastatic Prostate Cancer on the Rise Since Decrease in Cancer Screenings
Rational Surgical Solutions’ mCRPC Master Now Offered as Free Download


 August 10, 2022
August 10, 2022