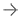ultronics将在EchoGo套件中提供人工智能驱动的EchoGo Pro作为应力-回波模块,以及EchoGo Core,后者是其用于自动收缩功能和应变分析的AI解决方案。EchoGo套件是一种基于云的服务,使用人工智能实现诊断路径的完全自动化,为临床医生提供近乎即时的报告,而不需要任何现场物理软件。
January 6, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Ultromics' EchoGo Pro, anartificial intelligence (AI)powered, outcomes-driven, decision support tool to automatically identify coronary artery disease (CAD) inechocardiograms.
CAD的迹象可能很微妙。误诊发生在五分之一的患者中,可能导致糟糕的治疗和结果,这是由临床医生之间的主观解释加剧。通过使用EchoGo Pro,临床医生可以从在英国牛津的大数据集上训练的人工智能模型中获得额外的见解。该技术改善了疾病预测,支持更准确的诊断。这将导致更早的适当干预,减少心脏事件和改善患者护理。
"Coronary artery disease is a huge global burden, affecting tens of millions of peoples' lives worldwide," saidRoss Upton, Ph.D., founder and CEO of Ultromics. "Heart disease is the biggest killer globally and this number is increasing daily. Our goal is to help doctors detect CAD more accurately, improving patient outcomes and saving lives."
Ultromics will offer EchoGo Pro as a stress-echo module in the EchoGo suite alongside EchoGo Core, its AI solution for automated systolic function and strain analysis. The EchoGo suite is a cloud-based service that uses artificial intelligence to fully automate the pathway to diagnosis, providing near-instant reports for clinicians without any need for physical software on site. By automating echocardiogram analysis as part of a cloud service, Ultromics hopes to save clinicians even more time when compared to traditional on-site solutions.
"COVID-19 has placed an even greater pressure on cardiac care and looks likely to have lasting implications in terms of its impact on the heart" explained Upton. "The healthcare industry needs to quickly pivot towards AI powered automation to reduce the time to diagnosis and improve patient care. To help support this shift and save countless lives we are making the EchoGo suite as complete and automated as possible to help clinicians rapidly assess disease and provide early, appropriate intervention. FDA approval for EchoGo Pro is the next step on our innovation journey to transform echocardiography and impact patients' lives"
EchoGo Pro is now clinically available in the European Union and U.S., and is being rolled out in the U.K. National health Service (NHS) as part of the prestigious NHSx award and assessed at Mayo Clinic.
Ultromics is a global health technology firm which provides autonomous echocardiography analysis through innovative AI solutions. The company developed the first fully automated solution for echocardiography and strain analysis. The platform, EchoGo, is a cloud-based AI service which delivers analysis to any vendor within minutes and with zero variability through its zero-click, full automation workflow. The technology was developed at the University of Oxford and built-in partnership with the NHS and has since raised over £20 million to help bring diagnostic quality to hospitals, improve patient care, and help make valuable resource and cost savings.
For more information:www.ultromics.com
Related AI Content:
How Will Artificial Intelligence Impact Healthcare?
FDA Clears AI-powered Cardiac Echo Analysis and Quantification by Ultromics
Mayo Clinic Uses Artificial Intelligence to Help Assess Cardiac Danger From COVID-19
Ultromics Earns Frost & Sullivan Innovation Award for Pioneering AI-based Cardiac Echo Software


 August 18, 2022
August 18, 2022